Fred Hoerndli
How is long-distance transport of AMPA receptors regulated?
Coni Stacher Hoerndli www.Chsciencedesign.com
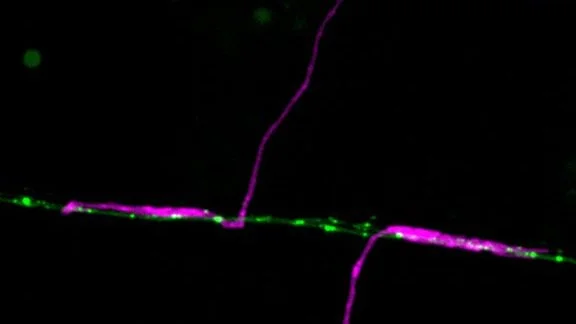
Using GFP tagged GLR-1 receptors (C.elegans homologues of GluR1), we have previously established a microscopy platform enabling us to directly follow receptor transport in an intact nervous system in C.elegans. This approach revealed that Glr-1 receptors were very mobile, transported out from the neuronal soma and between synapses in vesicles moved by the molecular motor Kinesin-1. (Hoerndli et al., 2013). Furthermore, this transport was actively regulated by neuronal activity through calcium and calcium calmodulin-dependent kinase II (CaMKII). Indeed, we showed that presynaptic neuronal inhibition led to decrease somatic export of GLR-1 vesicles which could be bypassed by constitutively active forms of CaMKII (Hoerndli et al., 2015). Finally, chromophore-assisted-light-inactivation (CALI) of CaMKII in the cell body or specific synapses revealed that CaMKII was required for Kinesin-1 dependent mobility of GLR-1 vesicles essential for synaptic function and maintnenance.
At CSU the Hoerndli Lab is now investigating how CaMKII and related molecular signaling pathways control synaptic delivery and removal of GLR-1. In particular, we are interested in the role of ROS signaling downstream of calcium entry and phosphatases downstream of CaMKII signaling events that could modulate synaptic GLR-1 transport and function.
DOES ROS SIGNALING MODULATE SYNAPTIC GLR-1 TRANSPORT AND INSERTION?
Work by Rachel Doser in the Hoerndli lab has now shown that mitochondrial ROS modifies the transport dynamics of GLR-1 receptors. Results published in the Journal of Neuroscience in 2021, have demonstrated that ROS levels negatively impact activity-dependent calcium levels in dendrites. Using genetic and pharmacological experiments, Rachel has shown that ROS affect calcium signaling downstream of the L-type voltage-gated channel Egl-19 in C. elegans. This directly affected the frequency of GLR-1 vesicle stops in the dendrites leading to a quantifiable decrease in GLR-1 delivery to synapses. Currently experiments in the lab have shown that synaptic mitochondria can uptake calcium from local synaptic activity. The mechanism of how this calcium uptake might provide a feedback loop for synaptic recruitment of GLR-1 is currently being investigated.
HOW DOES LAR-RPTP/PTP-3 REGULATE SYNAPTIC DELIVERY AND REMOVAL OF GLR-1?
Work by a former graduate student in the lab Dr. Dayton Pierce, has revealed a novel dual modulatory role for the phosphotyrosine phosphatase PTP-3, a homologue of LAR-RPTP, in regulating transport and synaptic retention of GLR-1 receptors. More specifically, Dayton has shown that the N-terminal 3 Ig-Like domain of the PTP-3A isoform modulates GLR-1 transport from the cell body and the C-terminal domain containing the phosphatase domains modulates retention of GLR-1 receptors at synapses. In addition, to demonstrating these functions Dayton also showed that these functions were cell specific and necessary in adult animals for proper function of GLR-1 synapses. These molecular functions are necessary for proper neuronal function, since behavioral assays in collaboration with the Stetak lab at the Biozentrum in Switzerland revealed that loss of PTP-3A led to impaired short and long-term associative learning of C. elegans that could be rescued by expressing PTP-3A postsynaptically. In addition, immunohistochemical analyses have shown that PTP-3A is cleaved in vivo in C.elegans neurons. These findings lead to an exciting new model for coordinating global cellular transport and synaptic retention of GLR-1 depicted below. This modele postulates that synaptic activity-dependent cleavage of PTP-3A leads to release of the N- and C-terminal domains both of which act in a coordinated fasion, the first to upregulate GLR-1 transport, the second to retain GLR-1 at synapses.